Using the nuclear module
The nuclear module provides the user with various type of nuclear data and can be useful in the interpretation and verification of gamma spectra. The module is constantly updated with new functions to help the user in various tasks.
The nuclear module uses various sources of data. Gamma energy transition have been obtained from the The Lund/LBNL Nuclear Data Search while decay chains and other data, such as decay branching ratios, have been taken directly from the radioactivedecay python pacakge.
Nuclide search and identification
In the previous tutorials we showed how a gamma spectrum can be loaded, calibrated and how the user can perform a peak search allowing the individuation of the main gamma lines.
Once the energy of a peak has been determined, we can try to assign it to a particular gamma emitter. To do so, the nuclear module provides a search_gamma_line function that allows the user to search for all the gamma emitters having gamma energies close to the detected one. To show how this can be done, let us consider the spectrum used in the previous tutorials as an example:
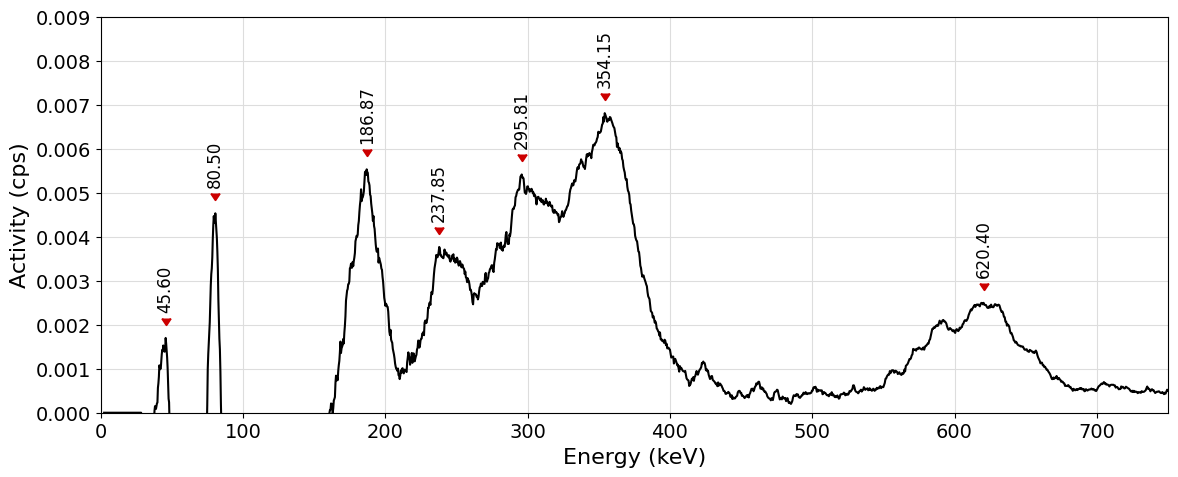
Let us look at the spectrum and try to identify the peak that has been located at \(186.87 \mathrm{keV}\). To do so, let us interrogate the search_gamma_line function by looking for gamma lines of energy centered around that energy with a range of \(\pm 1\mathrm{keV}\). This can be done as follows:
from pygammaspec.nuclear import search_gamma_line
gamma_lines = search_gamma_line(186.87, delta=1)
print(f"{len(gamma_lines)} possible gamma emission have been found\n\n")
123 possible gamma emission have been found
As can be seen 123 possible gamma emitters fall in the selected range. To help us out, let us apply some filering based on the nuclide halflife and gamma line intensity. This can be done acting directly on the halflife_threshold and intensity_threshold of the search_gamma_line function. Starting from the assumption that the sample has beeen acquired from a commonly existing item let us discard nuclides with short half-life smaller than, let’s say, \(60s\). Please notice how this hypothesis if valid only in the case of long-lived isotopes and would not work in the identification of those peaks associated with short lived decay daughters. Furthermore, given the small acitvity of the sample, let us exclude the low intensity bands, that would not contribute much to the spectrum, by setting \(1\%\) as the lowest intensity:
gamma_lines = search_gamma_line(186.87, delta=1, halflife_threshold=60, intensity_threshold=1)
print(f"{len(gamma_lines)} possible gamma emission have been found\n\n")
print("{:^8} {:^6} {:^10} {:<10}{:^6}".format("E(keV)", "I(%)", "t1/2(s)", "nuclide", "decay"))
print("-------------------------------------------------------")
for line in gamma_lines:
energy, intensity, decay, halflife, nuclide, _ = line
print("{:>8} {:>6} {:>10} {:<10}{:<6}".format(f"{energy:.3f}", f"{intensity:.2f}", f"{halflife:.2e}", nuclide, decay))
32 possible gamma emission have been found
E(keV) I(%) t1/2(s) nuclide decay
-------------------------------------------------------
185.930 1.74 5.04e+02 Au-187 e+b^+
186.000 7.00 1.80e+02 Pt-182 e+b^+
186.050 4.80 1.06e+05 Ac-226 e
186.150 1.30 8.46e+03 Pt-187 e+b^+
186.150 1.76 2.41e+04 Pa-234 b^-
186.170 9.40 6.35e+04 Au-193 e+b^+
186.200 8.50 8.10e+03 W-177 e+b^+
186.211 3.59 5.05e+10 Ra-226 a
186.274 3.37 1.98e+03 Ho-159 e+b^+
186.380 21.60 1.50e+03 Rn-221 b^-
186.400 3.30 1.75e+04 Hg-192 e
186.420 7.80 8.10e+03 W-177 e+b^+
186.500 3.11 4.23e+04 Bi-203 e+b^+
186.500 6.80 3.40e+02 Rn-206 e+b^+
186.680 2.23 3.91e+04 Pt-189 e+b^+
186.718 27.80 1.15e+04 Re-190m b^-
186.718 48.40 1.86e+02 Re-190 b^-
186.718 52.40 1.02e+07 Ir-190 e+b^+
186.718 66.30 1.17e+04 Ir-190m2 e+b^+
186.718 70.20 5.94e+02 Os-190m IT
186.770 3.00 6.78e+02 Ho-158 e+b^+
186.800 1.70 6.12e+02 Ce-131 e+b^+
186.800 5.00 3.90e+02 Os-179 e+b^+
187.000 37.20 1.28e+03 Ho-158m2 e+b^+
187.200 5.50 1.86e+02 No-255 a
187.220 4.30 1.48e+04 Tb-149 e+b^+
187.280 3.90 8.46e+03 Pt-187 e+b^+
187.400 1.66 3.78e+04 Ir-187 e+b^+
187.590 19.40 8.81e+06 Pt-188 e
187.600 16.50 1.57e+04 Bk-244 e+b^+
187.800 12.00 2.94e+02 Ta-169 e+b^+
187.870 1.26 5.31e+04 Y-86 e+b^+
Looking at the list of possible gamma emitters one can now proceed by exclusion discarding exotic elements and decay modes. Among the possible candidates \(^{226}\mathrm{Ra}\), deriving from the decay of naturally occurring \(^{238}\mathrm{U}\), appears to be the most plausible candidate with an energy of \(186.211\mathrm{keV}\).
Searching for decay daughters
In the previous section we assigned the peak at \(186.87\mathrm{keV}\) to the \(^{226}\mathrm{Ra}\) nuclide. Such a nuclide can decay forming a series of radioactive daughters that, possibly, can in turn be source of other gamma ray peaks. It is wise, as such, to verify if those peaks are present in the spectrum.
to do so, we can use the decay_products function of the nuclear module. The function returns all the possible decay producto of a given nuclide and stops only when a stable isotope has been detected. To use the function, the following syntax can be used:
from pygammaspec.nuclear import decay_products
daughters = decay_products("Ra-226", branching_ratio_threshold=0.01)
print(daughters)
['Ra-226', 'Rn-222', 'Po-218', 'Pb-214', 'Bi-214', 'Po-214', 'Pb-210', 'Bi-210', 'Po-210', 'Pb-206']
where we used the branching_ratio_threshold to discard all the branches with less than \(1\%\) probability.
Once a decay daughter has been found, possible gamma lines associated to the nuclide can be searched using the nuclide_gamma_lines function from the nuclear module. Let us proceed in order by considering the case of the Pb-214 nuclide (Po-218 is not a gamma emitter).
from pygammaspec.nuclear import nuclide_gamma_lines
energies, intensities = nuclide_gamma_lines("Pb-214")
print(f"A total of {len(energies)} gamma lines has been found")
print(f"Intensity range from {max(intensities):.2e} to {min(intensities):.2e}")
A total of 29 gamma lines has been found
Intensity range from 3.76e+01 to 4.00e-03
As can be seen a moltitude of transitions are possible for the nuclide. To obtain the most relevant gamma emissions we can filter by intensity either maually, by setting a threshold with the keyword intensity_threshold, or automatically by using the limit_intensity option. The latter automatically searches for the highest intensity transition for the nuclide and returns only those transition that are at least \(10\%\) of the maximum intensity. Using the latter option we can obtain the following result:
energies, intensities = nuclide_gamma_lines("Pb-214", limit_intensity=True)
print("E (keV) \tI (%)")
print("----------------------")
for energy, intensity in zip(energies, intensities):
print(f"{energy:.3f} \t{intensity:.2e}")
E (keV) I (%)
----------------------
241.997 7.43e+00
295.224 1.93e+01
351.932 3.76e+01
As can be seen, three transitions are possible for the \(^{214}\mathrm{Pb}\) and can in fact be associated to the three peaks immediately of the right of the \(^{226}\mathrm{Ra}\) peak.
Predicting the position of decay daughters peaks
What has been done in the previous example can also be automated using the decay_products_spectrum function of the nuclear module. The function, once given a father nuclide, will automatically search for all gamma lines associated to the decay chain. Filtering of the obtained gamma lines can also be applied directly from the function call using the branching_ratio_threshold,intensity_threshold and limit_intensity keywords. As an example, for the case of Ra-226 the following result can be obtained:
from pygammaspec.nuclear import decay_products_spectrum
nuclides, energies = decay_products_spectrum("Ra-226", branching_ratio_threshold=0.1, limit_intensity=True)
for nuclide, energy in zip(nuclides, energies):
# keep only energy within the recorded spectrum
if energy > 800:
continue
print("{:<10}{:>8} keV".format(nuclide, f"{energy:.3f}"))
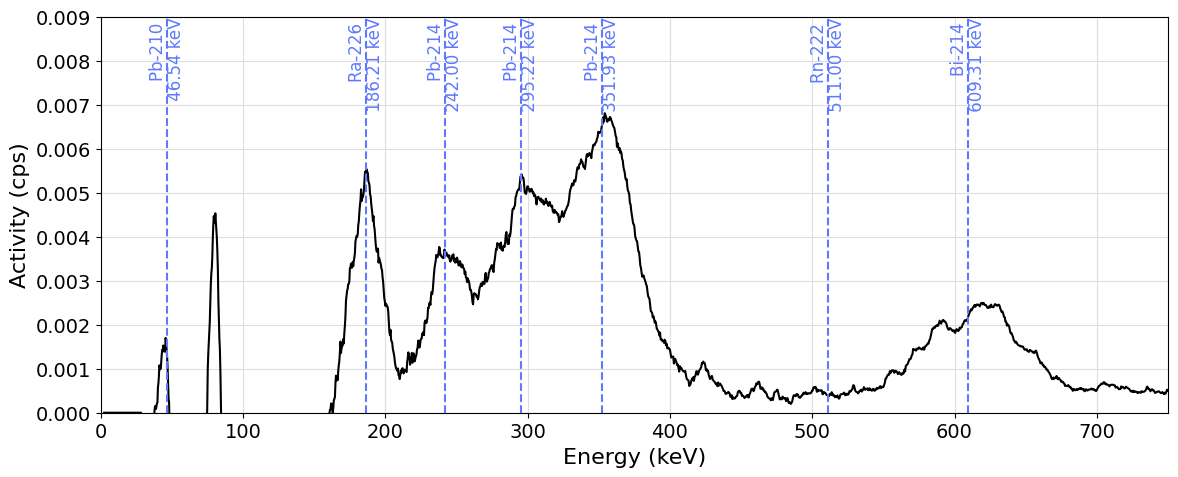
Ra-226 186.211 keV
Rn-222 511.000 keV
Pb-214 241.997 keV
Pb-214 295.224 keV
Pb-214 351.932 keV
Bi-214 609.312 keV
Bi-214 768.356 keV
Po-214 799.700 keV
Pb-210 46.539 keV
The result of the decay_products_spectrum function can also be examined graphically directly using the plot_spectrum function from the pygammaspec.visualization module. The function accepts a nuclide keyword that, when set, automatically provides some indication about the expected positions of the gamma lines of the various decay products:
from pygammaspec.spectrum import GammaSpectrum, Calibration
from pygammaspec.visualization import plot_spectrum
sample = GammaSpectrum.from_PRA_histogram("../utils/weak_radium.txt", 25851)
background = GammaSpectrum.from_PRA_histogram("../utils/background.txt", 25851)
calibration = Calibration.from_calibration_file("../utils/calibration.txt")
spectrum = (sample-background).average_smoothing(10)
spectrum.calibration = calibration
plot_spectrum(spectrum, enrange=(0, 750), yrange=(0, 0.009), nuclide="Ra-226")
X-ray emissions
The nuclear module also provides a simple interface to X-ray data listing a wide range of characteristic emissions associated with various elements.
Similarly to what already shown for the identification of gamma ray peaks, also X-ray emisison can be seached based on energy. This can be done using the function search_x_ray_line:
from pygammaspec.nuclear import search_x_ray_line
lines = search_x_ray_line(11.91, delta=0.1)
for line in lines:
print(line)
[11.812, 'Tl', 'L_{b6}']
[11.824, 'Hg', 'L_{b1}']
[11.826, 'As', 'K_{b5}']
[11.864, 'As', 'K_{b2}']
[11.867, 'As', 'K_{b4}']
[11.871, 'Np', 'L_{l}']
[11.878, 'Br', 'K_{a2}']
[11.896, 'Fr', 'L_{a2}']
[11.915, 'Hg', 'L_{b2}']
[11.916, 'Au', 'L_{b5}']
[11.924, 'Br', 'K_{a1}']
[11.931, 'Tl', 'L_{b4}']
[11.955, 'Re', 'L_{g6}']
[11.992, 'Hg', 'L_{b3}']
[12.009, 'Re', 'L_{g2}']
All the characteristic X-ray associated with an element can be listed using the element_x_ray_lines function according to:
from pygammaspec.nuclear import element_x_ray_lines
energies, shells = element_x_ray_lines("Au")
print("E (keV) Shell")
print("----------------")
for energy, shell in zip(energies, shells):
print(f"{energy:.3f}\t {shell}")
E (keV) Shell
----------------
8.494 L_{l}
9.628 L_{a2}
9.713 L_{a1}
10.309 L_{h}
11.160 L_{b6}
11.205 L_{b4}
11.442 L_{b1}
11.576 L_{b2}
11.610 L_{b3}
11.916 L_{b5}
13.382 L_{g1}
13.709 L_{g2}
13.731 L_{g6}
13.807 L_{g3}
66.372 K_{a3}
66.991 K_{a2}
68.806 K_{a1}
77.577 K_{b3}
77.982 K_{b1}
78.477 K_{b5}
80.130 K_{b2}
80.382 K_{b4}