Basic usage
The core of the pypH library is represented the Systemclass. This class represents a generic system of acid-base equilibrium reactions happening simultaneously within a solution. To define a System class object one should provide all the acid-base species and the eventually relevant spectator species. These are in turn represented in software by the Acid and Spectator classes.
The simple case of a single acid
Let us start discussing the working of the library by showing how the logarithmic diagram of a single acid species can be plotted. For this example let us consider a \(0.05M\) solution of oxalic acid.
The starting point of the operation is to define an Acid object that will represent our oxalic acid species in solution. The Acid class is a simple object capable of encoding a generic mono or polyprotic acid. An instance of the class can be created providing the \(pKa\) of the acid and its total concentration.
from pypH.acid import Acid
oxalic_acid = Acid([1.25, 4.14], 0.05)
Once an instance of the Acid class has been defined the concentration of each deprotonation product can be computed at a given \(pH\) using the concentration function. The user can select the desired deprotonation product using as index the number of protons removed from the base acid. As an example the concentration of the \(A^{2-}\) species (index = 2) at \(pH=5.2\) can be computed according to:
print(oxalic_acid.concentration(2, 5.2))
0.045993669391864306
Once the acid system has been defined it can be passed to the System class to generate the acid-base system representing the desired solution. Please notice how in this case the operation is trivial since the oxalic acid is the only species present in the solution. A System object however a more generic object that, as it will be shown further on in this guide, can support multiple Acid and Spectator objects and represents complex acid-base systems. Once the desired System object has been defined the corresponding logarithmic diagram can be easily plotted according to:
from pypH.system import System
plotter = System()
plotter.add(oxalic_acid)
plotter.plot_logarithmic_diagram(show_legend=True)
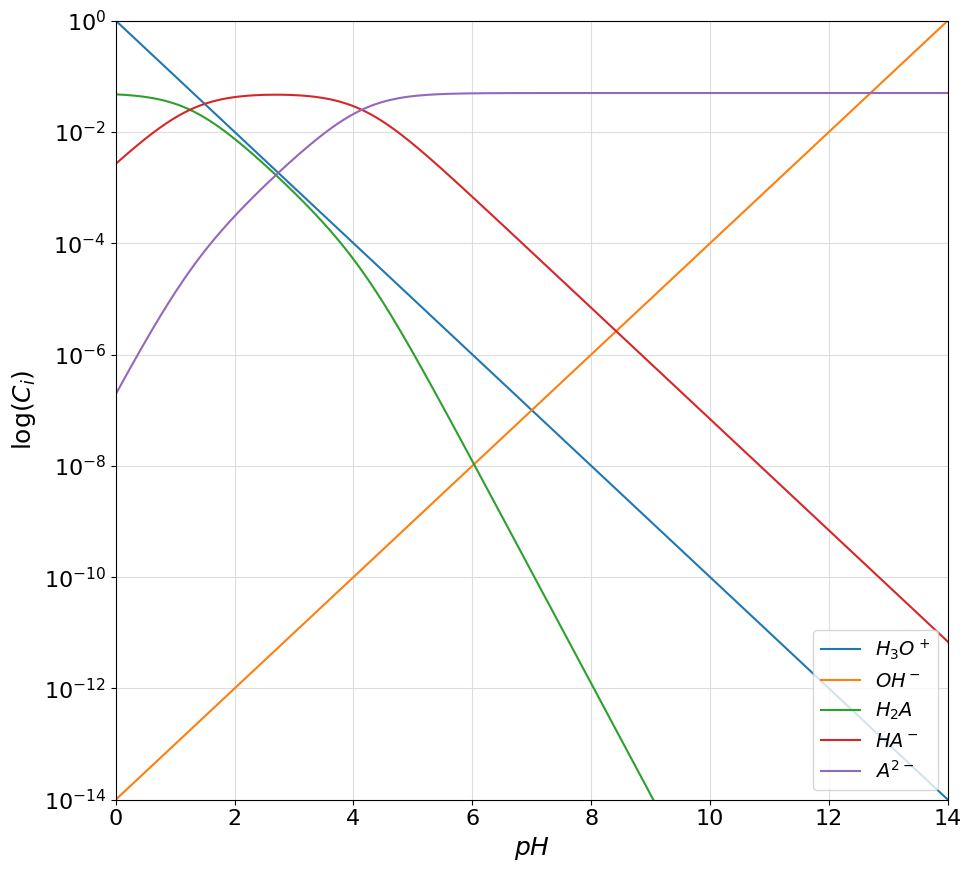
Please notice how the names of the deprotonation speces have automatically been set to \(H_2A\), \(HA^{-}\) and \(A^{2-}\) by the Acid class constructor. If desired, these labels can be customized by the user by directly listing the desired name in the class constructor. To do so, a list of strings, given in order from the completely protonated form to the completely deprotonated one, can be given as the names variable. LaTeX syntax is accepted. As an example, the previous plot can be customized as follows:
oxalic_acid = Acid([1.25, 4.14], 0.05, names=["$H_2Ox$", "$HOx^-$", "$Ox^{2-}$" ])
plotter = System()
plotter.add(oxalic_acid)
plotter.plot_logarithmic_diagram(show_legend=True)
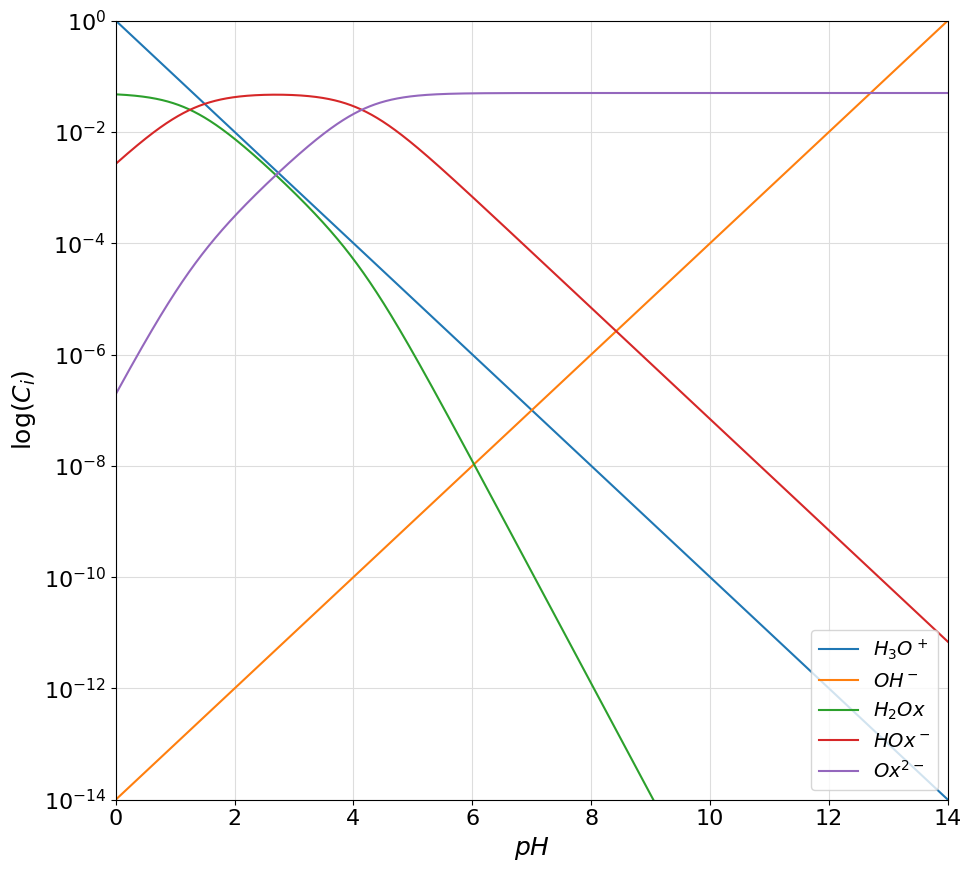
Using Spectator species
Besides acid-base active species the pypH library also allows the representation of spectator species and ions. This can be useful for example in the resolution of protonic balances of various systems (e.g. salts or strong acid or bases). This can be done using the the Spectator class. An object of the Spectator class can be initialized by specifying a name for the species and a concentration value. As an example the case of the dissociation of a \(0.01M\) hydrochloric acid solution can be represented on the logarithmic diagram as:
from pypH.spectator import Spectator
from pypH.system import System
chloride = Spectator("$Cl^-$", 0.01)
plotter = System()
plotter.add(chloride)
plotter.plot_logarithmic_diagram(show_legend=True)
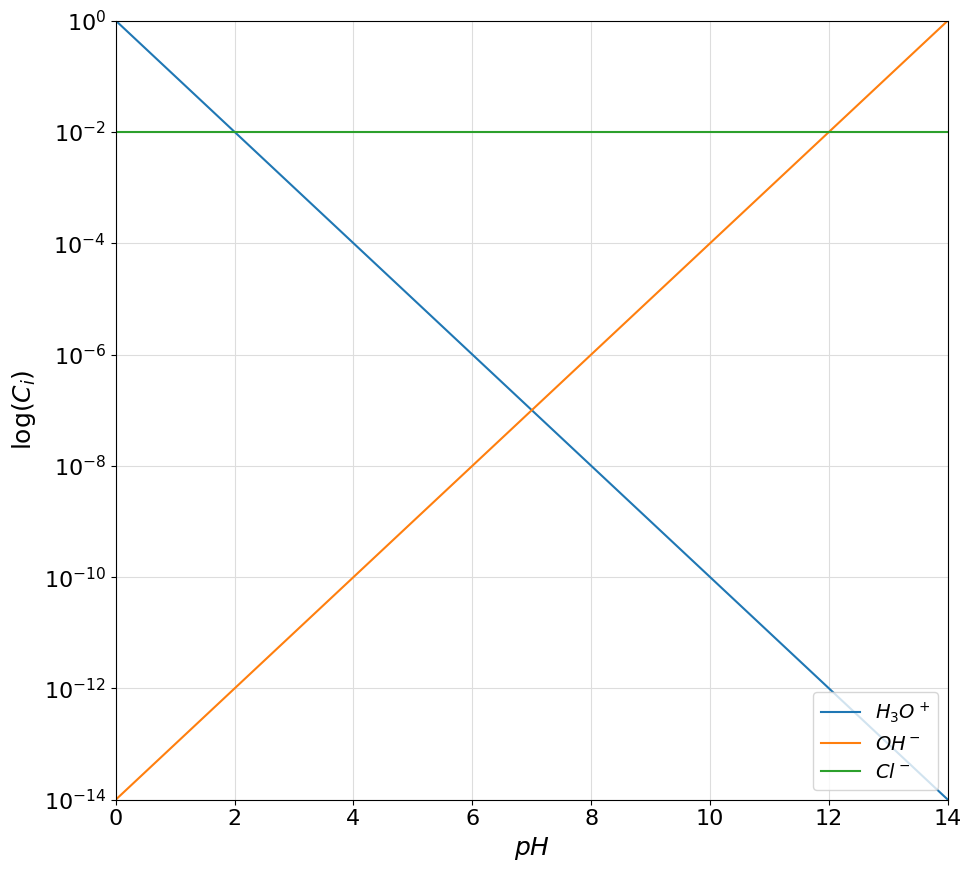
that, according to the protonic balance \([H_3O^+] = [OH^-] + [Cl^-]\), confirm the expected solution of \(pH=2\). Please notice how the add method of the System class automatically detects the type of term given to it (either Acid or Spectator object).